How To Calculate Midpoint Titration . The midpoint is reached when enough titrant has been released to allow half. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Locating the midpoint on a titration curve.
from www.numerade.com
Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. The midpoint is reached when enough titrant has been released to allow half. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Locating the midpoint on a titration curve.
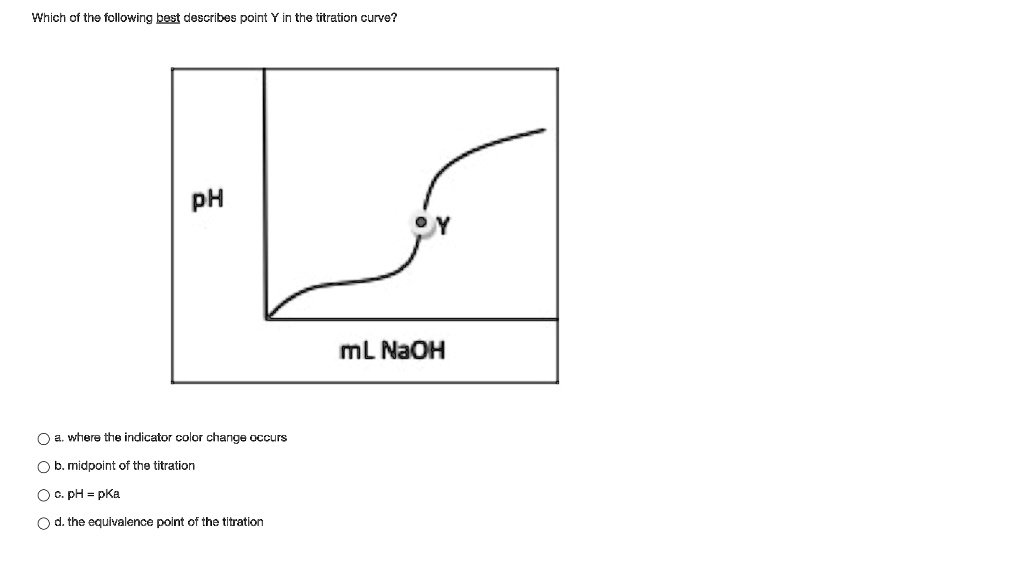
SOLVED Which of tha following best describes point in the titration
How To Calculate Midpoint Titration Locating the midpoint on a titration curve. Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The midpoint is reached when enough titrant has been released to allow half. By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation How To Calculate Midpoint Titration Locating the midpoint on a titration curve. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. The midpoint is reached when enough titrant has been released to allow half. For the titration of. How To Calculate Midpoint Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts How To Calculate Midpoint Titration The midpoint is reached when enough titrant has been released to allow half. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. For the titration of a weak. How To Calculate Midpoint Titration.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts How To Calculate Midpoint Titration For the titration of a weak acid with a strong base, the ph curve is initially acidic and. Locating the midpoint on a titration curve. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Before any titrant is added, the analyte solution. How To Calculate Midpoint Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts How To Calculate Midpoint Titration Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. By using a solution with a known molarity and a. How To Calculate Midpoint Titration.
From www.science.lu
Wie funktioniert Titration? How To Calculate Midpoint Titration By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph curve is initially acidic. How To Calculate Midpoint Titration.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube How To Calculate Midpoint Titration Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. The midpoint is reached when enough titrant has been released to allow half. By using a solution with a known molarity and a color indicator, we measure how. How To Calculate Midpoint Titration.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube How To Calculate Midpoint Titration Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. Locating the midpoint on a titration curve. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong. How To Calculate Midpoint Titration.
From www.youtube.com
17.2 AcidBase Titrations and Titration Curves General Chemistry How To Calculate Midpoint Titration By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. Before any titrant is added, the analyte solution is mostly the weak base,. How To Calculate Midpoint Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 How To Calculate Midpoint Titration Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. For. How To Calculate Midpoint Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube How To Calculate Midpoint Titration Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. The midpoint is reached when enough titrant has been released to allow half. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after. How To Calculate Midpoint Titration.
From www.vernier.com
AcidBase Titrations > Experiment 17 from Investigating Chemistry How To Calculate Midpoint Titration By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the. How To Calculate Midpoint Titration.
From mavink.com
Strong Acid And Base Titration Curve How To Calculate Midpoint Titration You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions except “after equivalence” (where the ph is set by excess titrant, which. Locating. How To Calculate Midpoint Titration.
From www.writework.com
Titration of amino acids WriteWork How To Calculate Midpoint Titration Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. By using a solution with a known molarity. How To Calculate Midpoint Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free How To Calculate Midpoint Titration The midpoint is reached when enough titrant has been released to allow half. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Locating the midpoint on a titration curve. For the titration of a weak acid with a strong base, the ph. How To Calculate Midpoint Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Calculate Midpoint Titration By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. Locating the midpoint on a titration curve. The midpoint is reached when enough titrant has been released to allow half. You can use this same approach to calculate the titration curve for the titration of a weak base. How To Calculate Midpoint Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors How To Calculate Midpoint Titration The midpoint is reached when enough titrant has been released to allow half. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Locating the midpoint on a titration curve. Before any titrant is added, the analyte solution is mostly the weak base,. How To Calculate Midpoint Titration.
From www.myxxgirl.com
Where Does The Equivalence Point Occur On A Titration My XXX Hot Girl How To Calculate Midpoint Titration Before any titrant is added, the analyte solution is mostly the weak base, ammonia,. By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the. How To Calculate Midpoint Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free How To Calculate Midpoint Titration By using a solution with a known molarity and a color indicator, we measure how much of the solution is required to. For the titration of a weak acid with a strong base, the ph curve is initially acidic and. Titrations where the analyte is a weak base or acid will need equilibrium / ice table calculations in all regions. How To Calculate Midpoint Titration.